
Means of the most recent email address, if any, provided by such party to Varsity Tutors. Infringement Notice, it will make a good faith attempt to contact the party that made such content available by If Varsity Tutors takes action in response to Information described below to the designated agent listed below. Or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one To traverse the hydrophobic interior of a cell membrane, a molecule should be nonpolar therefore, molecule A will have an easier time traversing the membrane. Water is a highly polar molecule therefore, polar molecules will dissolve more easily in water (in our case, molecule B). Since in molecule B has the higher electronegativity, molecule B will be more polar (larger electronegativity difference) than molecule A. The higher the electronegativity difference, the more polar. The polarity of a molecule is calculated using the electronegativity differences between the atoms in the molecule.
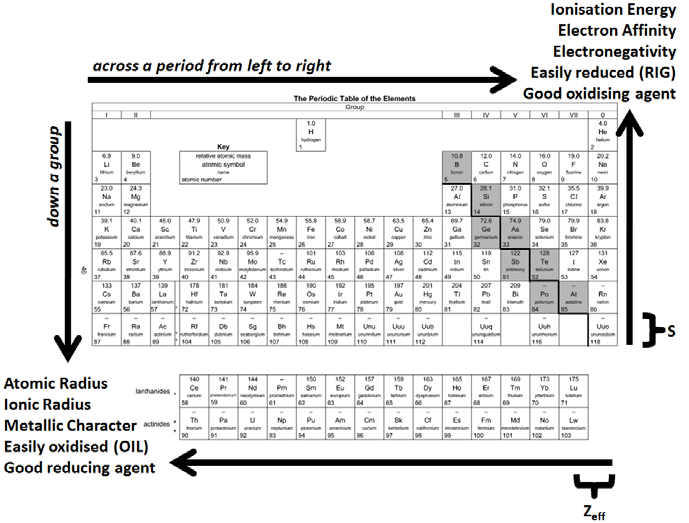
This means that has the higher electronegativity. The question states that in molecule B is at the top right. For example, fluorine (the most top-right, non-noble gas element on the periodic table) has the highest electronegativity (note that noble gases have no reactivity, so electronegativity is not measured for them). Recall that electronegativity increases as you go towards the top and right of the periodic table. To answer this question, we need to look at the electronegativity periodic trend.


 0 kommentar(er)
0 kommentar(er)
